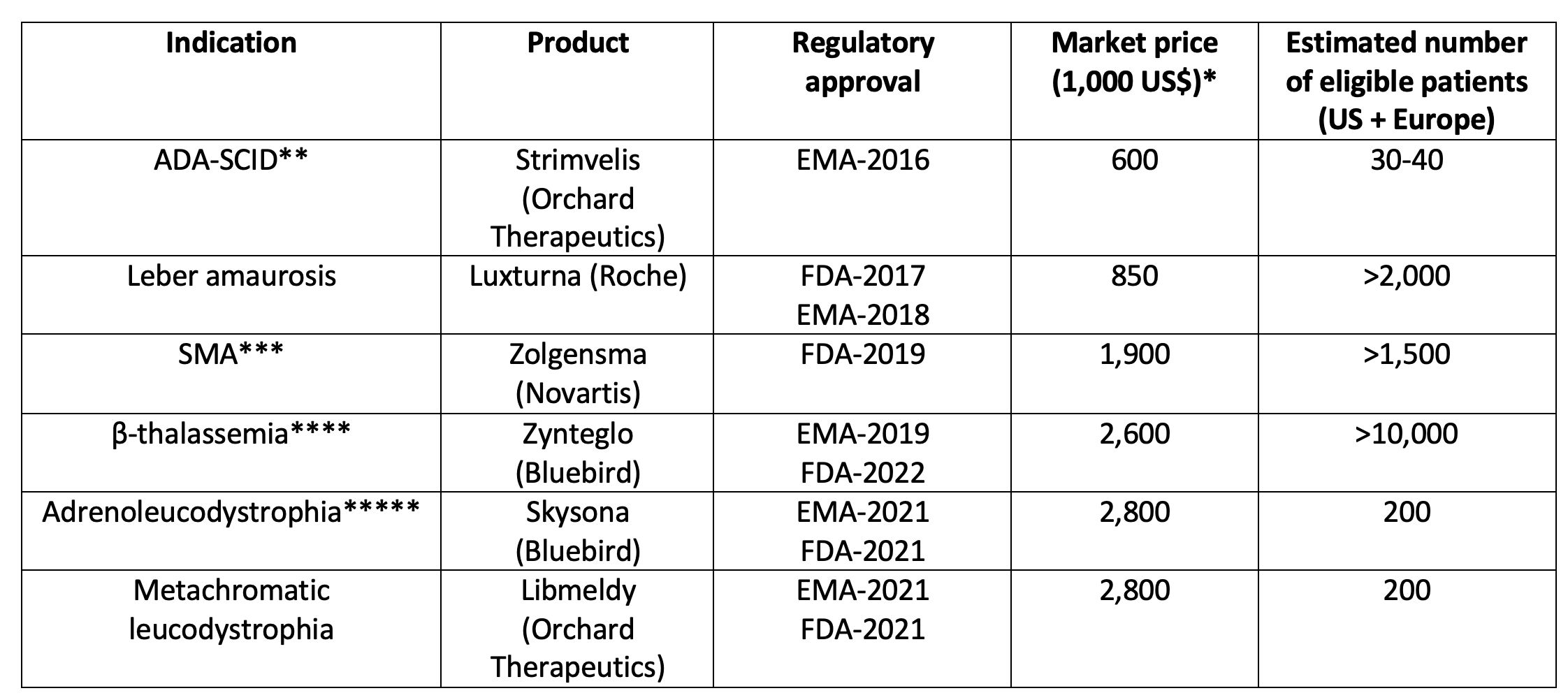
Over the last 10–15 years, advances in genomic research have led to significant innovations in medicine, notably in the field of rare diseases. New drugs to treat cystic fibrosis and biotherapeutics for hemophilia are among many salient examples. Unfortunately, these innovative drugs are extremely costly (for instance €200,000–300,000 per patient per year for the treatment of cystic fibrosis). The new field of gene therapy as illustrated in Table 1 is particularly striking.
Table 1 Some approved gene therapy products in the US by the Food and Drug Administration (FDA) and in Europe by the European Medicines Agency (EMA)
Notes: * Price on the US market except for Strimvelis that has been marketed in Europe only. ** Severe combined immunodeficiency caused by adenosine deaminase deficiency. Strimvelis has since been withdrawn from the market. *** Spinal muscular atrophy. **** Approved for non β0/ β0 patients, older than 12 years and without HLA-genoidentical donor. Zynteglo has since been withdrawn from the market in the EU. ***** Skysona has since been withdrawn from the market in the EU.
In its official strategy documents, the European Commission (2021) has recognised this challenge, and has stressed the need to ensure access to affordable medicines for patients and to address unmet medical needs, in the areas of antimicrobial resistance and rare diseases in particular. In this respect, the Commission has stressed four directions : (i) enhancing competition; (ii) working with national authorities to exchange information on sustainable health systems, pricing, cost-effectiveness, payment, procurement policies and affordability ; (iii) enhancing transparency through guidelines on how to calculate the R&D costs of medicines; and (iv) using the yearly European Semester cycle of economic policy coordination to assess national health systems and issuing country-specific recommendations to ensure their accessibility, efficiency and sustainability.
These useful avenues can be complemented by taking on board a suggestion of ours (Fischer et al. 2019) to reduce the price–cost margin earned by industry by asking them to create ‘benefit corporation divisions’ for rare diseases. The legal idea behind the benefit corporation concept is that it explicitly recognises that management should not solely pursue profit/stock market value as an objective but also common-good objectives, thereby protecting management against being sued by shareholders. Such benefit corporations could then have as explicit objective to negotiate with payers reasonable pricing clauses – clauses whose implementation could be facilitated by the push for transparency of the Commission mentioned above.
This idea could make a significant difference since, as documented by Thakor et al. (2015), the average risk-adjusted return earned by pharmaceutical companies has for decades exceeded the overall risk-adjusted stock market return by 3% a year. This is consistent with the widespread feeling that the shareholder value ‘model’ has gone too far and that environmental and social goals, accompanied by a proper governance (in short, ESG), have to become part of companies’ objectives in all sectors of the economy. The key, however, is to make these goals precise enough in order to be able to accurately monitor progress. In the pharmaceutical sector, making sure that companies earn a ‘reasonable’ return – i.e. competitive but no higher than that – is central, especially at a time where public budgets are under great stress. The fact that the public sector pays for these therapies should give it bargaining power to push for adopting the benefit-corporation legal form, which should pursue reasonable pricing.
Moving companies to endorse the benefit corporation legal form can take time, however, even if this legal form is enjoying a growing success. In this respect, one should note that the Covid-19 crisis pleads for an additional complementary instrument to favour affordable prices, namely, the centralisation of the purchase of rare disease therapies at the EU level.
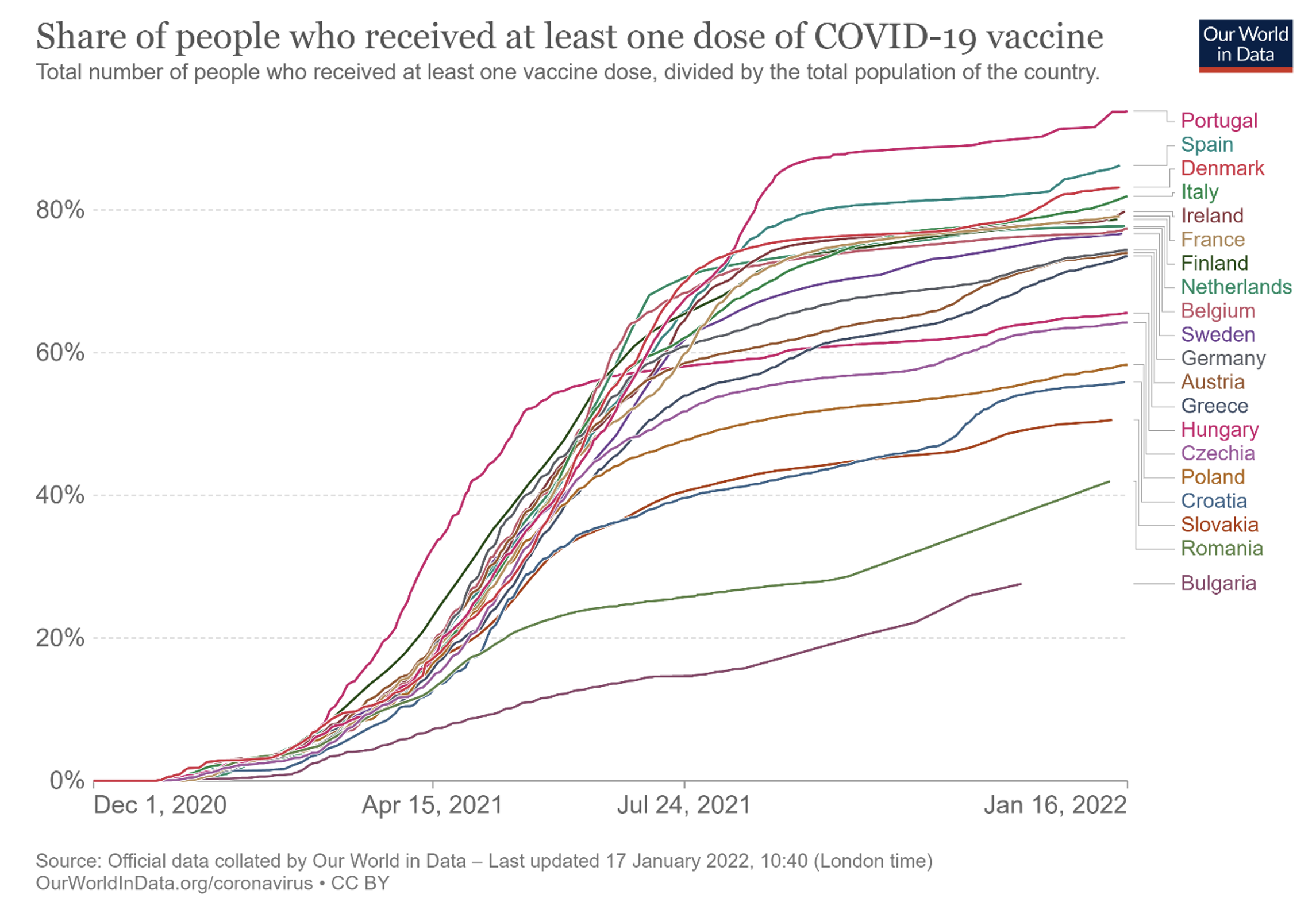
The Commission did play a crucial role in guaranteeing equitable access to covid vaccines among the 27 member states by taking over the role of central purchaser, which allowed most EU countries to progress with vaccination at similar rates (except for a small number of east European countries) until they hit their respective vaccine hesitancy constraints (see Figure 1 and Dewatripont 2021, 2022 for details).
This was better than the earlier approach, when only a few countries (namely Germany, France, Italy and the Netherlands) had teamed up, before accepting EU coordination. This action has therefore been seen as a success, which is why the Health Emergency Preparedness and Response Authority (HERA), created by the EU as a response to the Covid-19 pandemic, has been tasked to buy monkeypox vaccines for most EU countries.
Figure 1
Note also that the Commission did manage to obtain pretty low prices for covid vaccines: less than €4 for two doses of the AstraZeneca vaccine, €24 for two doses of the Pfizer/BioNTech vaccine, and €36 for two doses of the Moderna vaccine in the original contracts signed by the Commission (these numbers are contractually meant to be secret but were disclosed in a tweet by the Belgian secretary of state for budget).
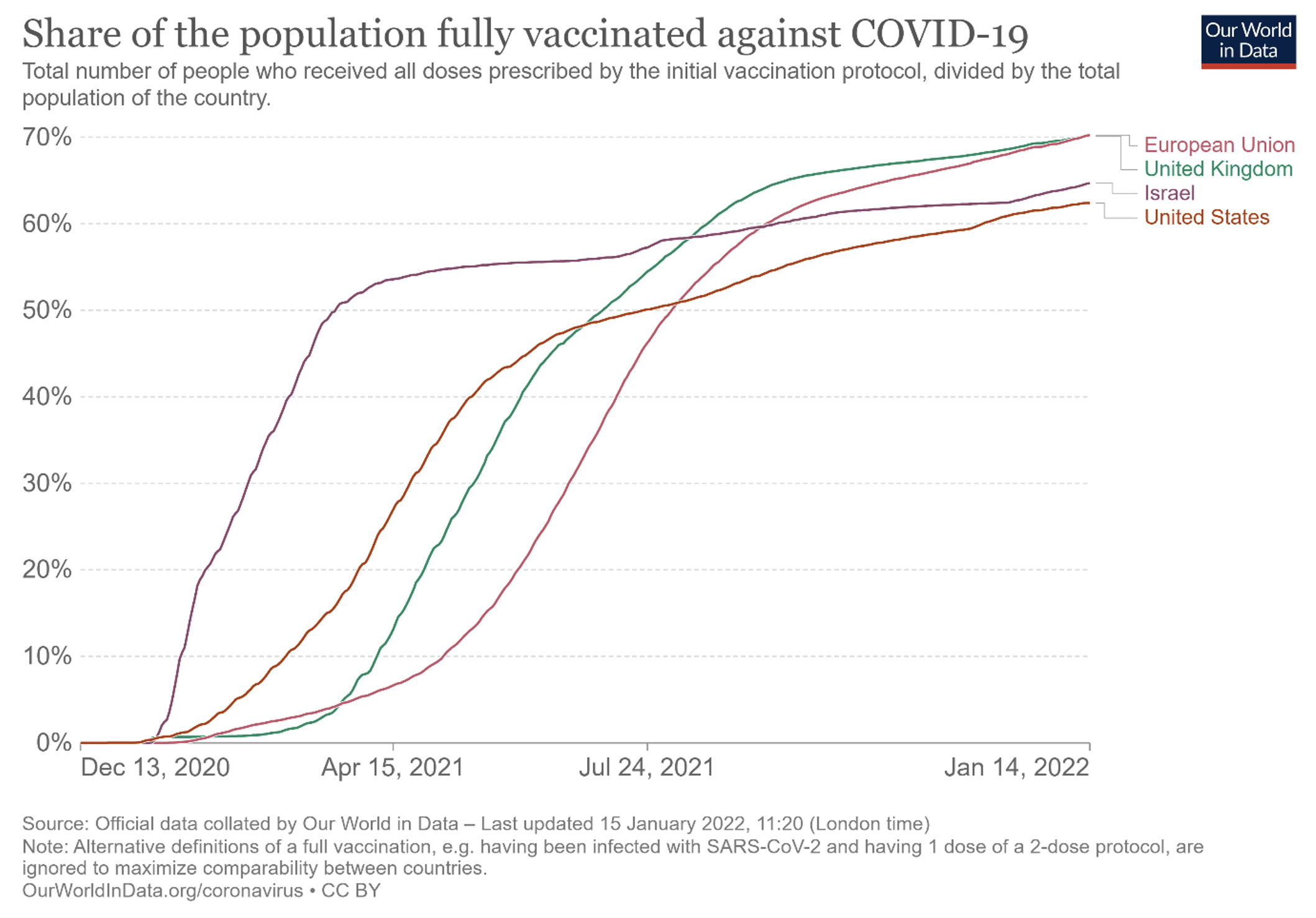
There is some truth to the criticism that the Commission has insisted too much on low prices at the expense of speed of delivery for a disease where speed was crucial given the economic cost of virus circulation; see Figure 2, and compare with the case of Israel in particular, which seems to have paid between $47 and more than $100 – respectively around €38 and €81 at the time – for two doses of the Pfizer vaccine (Winer 2021). The case of rare diseases is very different, however. Here, speed is less crucial than for an epidemic which can take off in sudden waves, while the ability to purchase at reasonable prices is a critical objective
(keeping prices ‘market consistent’, of course). The goal should be to reduce as much as possible ‘excessive’ profits, not to impose losses, since this would not be consistent with private-sector provision.
Figure 2
In this respect, we observe that many European countries have felt the need to try and ‘join forces’ when they bargain with industry over drug prices, forming the Beneluxa initiative for Benelux countries, Austria, and Ireland; the Valletta group for southern Europe; the Visegrad group; and the Nordic pharmaceutical forum (e.g. Bruce 2021). It would be natural to merge such initiatives and join forces at the EU level by inviting all EU member states to participate (this would mean convincing Germany and France, in particular). This would strengthen buyer power in order to balance seller power, in a market where, as documented academically (e.g. Kyle 2007), pharmaceutical companies put countries in competition against one another, with higher prices being ‘rewarded’ with earlier accessibility (just like Pfizer/BioNTech did with Israel for Covid vaccines). Coordinating purchases would improve bargaining power on the buyer side as far as this trade-off between prices and speed of access is concerned.
Rare diseases would moreover be a natural area for EU-wide intervention. One objective reason for high prices is of course the limited ‘market size’ in each country, which would therefore have an extra incentive to join pan-EU negotiations. A pan-EU purchase would also offer the prospect of higher sales, thereby making lower prices more economically sustainable for industry. One could even envisage ‘advance market commitments’ like with vaccines (e.g. Levin et al. 2022), ideally coupled with a percentage of profits to be refunded by the company in case these turn out to be higher than expected.
Finally, EU-wide coordination would also make sense in terms of the organisation of statistically significant clinical trials, which does represent a key challenge for rare diseases. And the same is true for the necessary coordination of national R&D funding beyond EU funding, along the lines of US funding for the National Institutes of Health. Once again, rare diseases would be a natural area for such pan-European coordination in order to maximise synergies.
References
Bruce, F (2021), “Europe’s Biggest Multi-Country Access Alliance Picks Up The Pace,” Pink Sheet – Informa Pharma Intelligence, 28 July
Dewatripont, M (2021), “Covid Vaccination Experiences”, VoxEU.org, 1 October.
Dewatripont, M (2022), “Which Policies for Vaccine Innovation and Delivery in Europe?”, International Journal of Industrial Organization, forthcoming.
European Commission (2021), Making Medicines More Affordable.
Fischer, A, M Dewatripont and M Goldman (2019), “Benefit Corporation: A Path to Affordable Gene Therapies?”, Nature Medicine 25: 1813-1814.
Kyle, M (2007), “Pharmaceutical Price Controls and Entry Strategies,” Review of Economics and Statistics 89: 88–99.
Levin, J, M Kremer and C Snyder (2022), “Designing Advance Market Commitments for New Vaccines,” Management Science 68: 4786-4814.
Sagonowsky, E. (2021), “Pfizer Eyes Higher Prices for COVID-19 Vaccine After the Pandemic Waves: Exec, Analyst,” Fierce pharma, February 23.
Thakor, R, N Anaya, Y Zhang, C Vilanilam, K Siah, C Wong and A Lo (2015), “Just How Good an Investment is the Biopharmaceutical Sector?,” Nature Biotechnology 35: 1149-1157.
Winer, S (2021), “Israel Has Spent $788m on Vaccines, Could Double Sum — Health Ministry,” The Times of Israel, 15 March.