Off-label uses of drugs, which occurs when a patient uses an active ingredient outside the label that it has been approved for by the US Food and Drug Administration (FDA), is a significant public health burden. Depending on disease area and study setting, the proportion of drugs used off-label in ambulatory care ranges from 21% to 44% (Blankart and Lichtenberg 2022, Bradford et al. 2018, Tunҫel 2020, Molitor 2012, McKibbin 2020). Paediatric conditions, cancer care, and rare diseases often have higher exposure to off-label use. To some patients, off-label use may be a last resort, especially when there are few approved treatment options. The problem with off-label use is that studies suggest that the vast majority of off-label uses lack scientific support: 61‒84% in ambulatory care (Radley et al. 2006), and 48% of choices in intensive care unit settings (Lat et al. 2011). Off-label use is therefore unlikely to be effective, and may even harm the patient.
Given that off-label use may waste resources and harm patients, it is important to determine what drives it and what the consequences for society are. A controversy arises around the role of pharmaceutical detailing (a marketing technique pharmaceutical companies use to educate a physician about a vendor's products) and the promotion of off-label uses that are not supported by (strong) scientific evidence. On regulatory grounds, promotion of off-label uses is illegal. Nevertheless, some pharmaceutical manufacturers have faced substantial regulatory settlements when allegations of off-label promotional activities were made (Shapiro 2018).
Could detailing activities lead to increased inappropriate use of drugs outside their designated labels? Answering this question is important from a policy perspective. If detailing of new drugs leads physicians to use drugs outside their designated label more often, despite the fact that promoting off-label uses is illegal, then the appropriateness of detailing physicians is put into question. Alternatively, measures such as anti-detailing or physician education could be modified to educate physicians about the areas where common off-label use are likely to cause no harm, but potentially cause significant waste through healthcare use and disability resulting from inappropriate use of drugs. Such policies, for example within the scope of the Centers for Medicare and Medicaid Services (New York Times 2009), would require independent review boards to develop unbiased and comprehensive evidence reviews independent from financial interests.
The evidence on the effects of detailing on off-label uses of drugs is controversial and concentrates on the use of anti-depressants. In an evaluation of anti-detailing policies of academic medical centres, Larkin et al. (2014) demonstrated that the presence of such policies reduced off-label use of antipsychotic drugs in children and adolescents by 11% compared to centres that did not have such a policy between 2006 and 2009. This finding suggests that pharmaceutical sales representatives potentially promote drugs that are not approved for paediatric use. However, the study further shows that responses to on-label uses of drugs are double the identified effect of off-label uses (34% decrease). Off-label use of drugs that were not promoted in pharmaceutical detailing activities increased by 35% as well. Besides, the study did not examine whether the academic medical centres that implemented an anti-detailing policy had higher off-label use to begin with – a source of biased effect estimate the study did not resolve on methodological grounds. Another study by Shapiro (2018) evaluated the impact of two informational shocks regarding the side-effect profiles of drugs frequently used off-label. AstraZeneca was sued for unlawfully promoting off-label use of the drug Seroquel and paid a fine of $520 million. Although the study found that new information about side effects was primarily transmitted through promotional activity, the study also found that detailing did not substantially increase off-label use. If anything, the distribution tilted from off-label towards on-label use.
In a recent study (Blankart and Lichtenberg 2022), we explore the effect of using a drug off-label on health care use and disability. We examine market size and innovation status measured by FDA approval year as attributes that lead to more or less off-label use at the condition level. In contrast to previous investigations of economic- and health-related consequences of off-label use that have emphasised cancer care and depression, our study covers a wide range of 247 conditions. We carefully account for the fact that some individuals may use off-label treatments more often than others and that some conditions lead to more intense use of healthcare and to disability. Additionally, we deal with an important endogeneity issue by demonstrating that individuals with higher disease severity and multiple conditions do not experience higher off-label use once we account for unobserved individual effects.
The data we rely on include combinations of prescription drug labels and indicated conditions for over 200,000 individuals in the US non-institutionalised population between 1996 and 2015, linked to comprehensive data from a pharmaceutical reference database. The data that match approved indications to active pharmaceutical ingredients that we use are unique as these are independent from medical compendia. We observe about 13,000 combinations by active ingredient and condition approved in France for prescription use from an independent source, edited by the French Centre National Hospitalier d’information sur le Médicament (Husson 2008). We linked those data to active ingredient-condition pairs reported by patients in the US Medical Expenditure Panel Survey. We measured off-label use as the fraction of drugs prescribed for a condition by individual according to condition-active ingredient pairs not recorded as on-label in our pharmaceutical reference data.
The effect of off-label use on healthcare use and disability
We found that off-label use generally increases medical expenditures and work loss cost. Our empirical estimates of two-way fixed effects regressions and counterfactual analyses suggested that using only on-label drugs compared to the average 2015 off-label drug use would lead to savings of $515 per individual in terms of healthcare use and work-loss cost. This value reflects 12.9% of all medical expenditure covered in our data. Estimates are robust across a number of specifications, for example when we exclude FDA approval year or include time fixed effects.
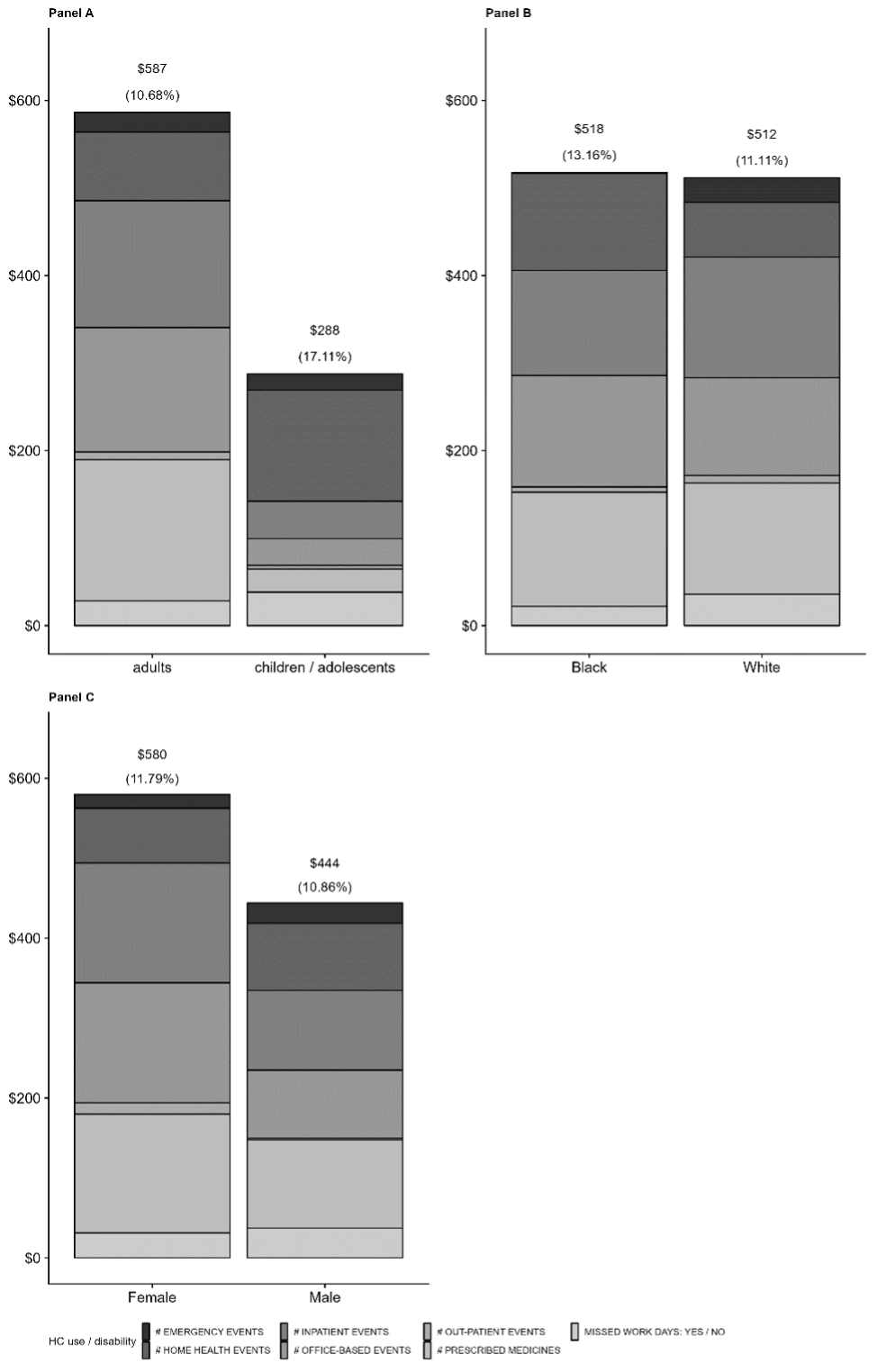
The dollar value of eliminating off-label use was based on seven outcomes of healthcare use and disability by person and condition. Estimates are larger in children and adolescents compared to adults, in females compared to males, but not in Black compared to white people (Figure 1). The difference in the cost of off-label compared to on-label treatments ($680 on average) does not outweigh the increased spending from off-label use. Off-label treatments as such are on average more expensive. The projected savings from reducing off-label use can be used as reference value for policy design to target reductions in off-label use.
Figure 1 Healthcare use and disability attributed to off-label use, by population groups, 2015
Off-label use, vintage, and implications for pharmaceutical detailing
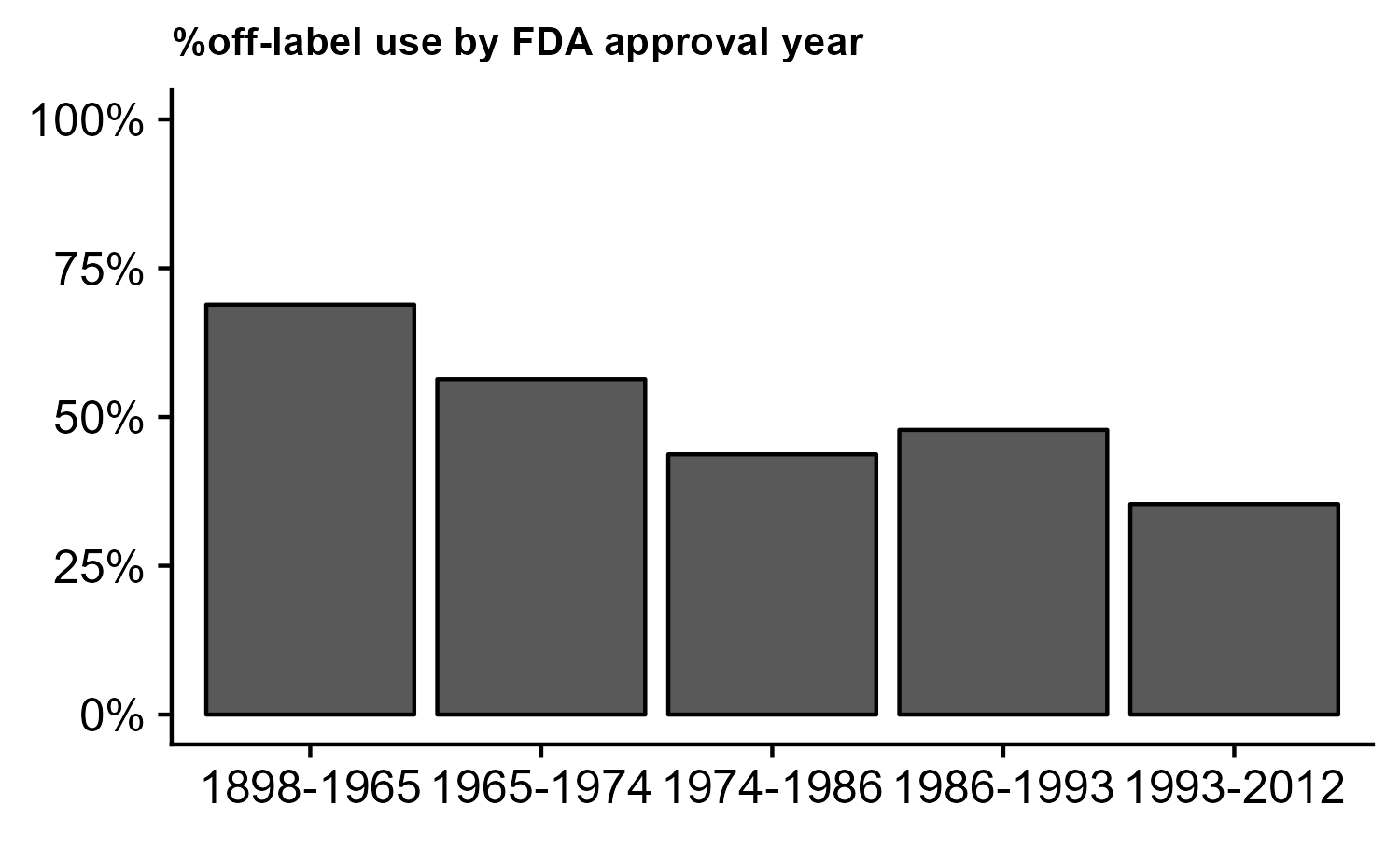
In the context of pharmaceutical detailing, our analysis demonstrates that off-label use is inversely related to year of regulatory approval – an indicator of a drug’s vintage. Figure 2 shows associations of off-label use across the spectrum of FDA approval years in five groups. The fraction of drugs used off-label that were approved before 1966 is about twice as high as that of drugs approved between 1993 and 2012: 68.5% compared to 35.4%. Pharmaceutical innovation may therefore have both direct effects on healthcare use and disability and indirect effects via the propensity to use drugs off-label. An explanation for these stark differences lies in that pharmaceutical detailing is most intense for the latest approved drugs. Duflos and Lichtenberg (2012) found that marketing expenditure declines by about 50–60% in the years immediately following generic entry.
Figure 2 Association of off-label use and FDA approval year
Summing up
Off-label use of drugs not only causes adverse drug events but also significant amounts of medical expenditure and work-loss cost to society. Although we cannot measure the effect of detailing on off-label use directly, considering approval status of a drug suggests that pharmaceutical detailing for which the majority of spendings by pharmaceutical manufacturers is made may not be the source of the substantial proportion of off-label use we and others have identified. Instead, if anything, pharmaceutical detailing may reinforce the evidence base according to regulatory information of the latest available treatments. Appropriate use of older drugs may be the more important target of anti-detailing or physician education policies. Such policies would require comprehensive evidence review of off-label uses of older drugs to guide prescribers towards the best available evidence and treatments. Whether such efforts are worth the savings potential we identified from eliminating off-label use is a question of policy implementation.
References
Blankart, K E and F R Lichtenberg (2022), “The Effects of Off-Label Drug Use on Disability and Medical Expenditure”, NBER Working Paper 30440.
Bradford, W D, J L Turner, and J W Williams (2018), “Off-Label Use Of Pharmaceuticals: A Detection Controlled Estimation Approach”, The Journal of Industrial Economics 66(4): 866–903.
Duflos, G, and F R Lichtenberg, (2012), “Does Competition Stimulate Drug Utilization? The Impact of Changes in Market Structure on US Drug Prices, Marketing and Utilization”, International Review of Law and Economics 32(1): 95–109.
Larkin, I, D Ang, J Avorn, and A S Kesselheim (2014), “Restrictions on Pharmaceutical Detailing Reduced Off-Label Prescribing of Antidepressants and Antipsychotics in Children”, Health Affairs 33(6): 1014–23.
Lat, I, S Micek, J Janzen, H Cohen, K Olsen, and C Haas (2011), “Off-Label Medication Use in Adult Critical Care Patients”, Journal of Critical Care 26(1): 89–94.
McKibbin, R J (2020), “The Effect of RCTs on Demand for Off-Label Cancer Drugs”, SSRN Scholarly Paper 3574623.
Molitor, D P (2012), “Physician Behavior and Technology Diffusion in Health Care”, thesis, Massachusetts Institute of Technology.
New York Times (2009), “Medicare and ‘Off-Label’ Cancer Drugs”, Opinion, 10 February.
Radley, D C, S N Finkelstein, and R S Stafford (2006), “Off-Label Prescribing Among Office-Based Physicians”, Archives of Internal Medicine 166(9): 1021–26.
Shapiro, B T (2018), “Informational Shocks, Off-Label Prescribing, and the Effects of Physician Detailing”, Management Science 64(12): 5925–45.
Tunҫel, T (2020), “Should We Prevent Off-Label Drug Prescriptions? Empirical Evidence from France”, SSRN Scholarly Paper 3694632.